Computer Systems Validation in an ERP Implementation Project
For regulated industries such as those in the life sciences sector, computer systems need to be validated to prove regulatory compliance. When implementing Enterprise Resource Planning (ERP) systems, companies must make sure that their validation plans form an integral part of the overall implementation project plan.
Validation is defined as “establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications and quality attributes”.

Properly validated processes and systems are a prerequisite to doing business in most of the world’s markets for pharmaceutical products. Failure to validate can lead to observations and warning letters from Regulators such as the Food & Drug Administration (FDA) during inspections, and in cases of severe deviations the company may be forced to withdraw its products from the market completely.
Since the early 1990s, a framework has developed called Good Automated Manufacturing Practice (GAMP®) which is designed to help companies achieve validated and compliant automated systems. The GAMP5®approach, which is the current version, aims to produce systems which meet all current healthcare regulatory requirements and expectations. It builds upon industry best practice and can easily be tailored to apply to manufacturing, clinical, laboratory and distribution environments.
When developing the ERP implementation plan it is very important that ERP validation effort is built in from the outset. A written validation master plan must be produced which:
- Describes the areas of the company within which the validation is to take place
- Describes in detail how the validation is to be performed for specific systems
- Forms the basis upon which the project progress and status will be reported.
An overview of the validation framework can be seen in the diagram below:
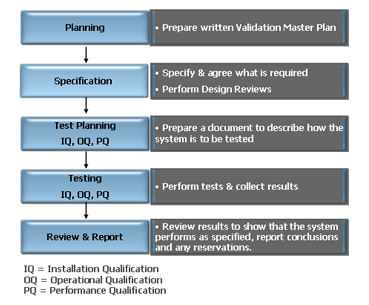
Computer Systems Validation Overview
GAMP® is a practical and pragmatic approach which facilitates the interpretation of regulatory requirements and promotes a system lifecycle approach based on best practice. It also helps to clarify roles and responsibilities within the project.
The benefits of using the GAMP® framework are:
- It helps deliver systems which are in line with user requirements, fit for purpose and compliant with regulatory requirements.
- It reduces the cost and time taken to achieve and maintain compliance.
- It provides a common language and terminology for the company and the Regulators.
- It provides the necessary documentary evidence to prove compliance and supports audits by customers and the Regulators.
- It improves operational efficiency with reduced risk of failure.
So, GAMP® is not a regulatory standard or a prescriptive implementation methodology, it is a framework, which when applied with good judgement and expertise can offer a robust and cost effective solution to the computer systems validation effort within an ERP project.
This blog was written by Frank Crewe, Principal Consultant. If you would like further information on Computer Systems Validation in ERP Implementations please send an e-mail to Frank Crewe.


